Thanks to its high acid strength and environment friendliness, a heteropoly acids (HPA) are widely used as heterogeneous catalysts. Of them, Keggin 12-H3PW12O40 is the one with strongest acid strength. However, because of the lack of effective characterization technology, some fundamental issues about the acid, such as the location and acid strength of acid protons, are controversial.
Teaming up with colleagues from US University of Virginia and the CAS Dalian Institute of Chemical Physics,a research team headed by Prof. DENG Feng from the CAS Wuhan Institute of Physics and Mathematics has made progress to settle the debate, laying a solid foundation for further application and modification.
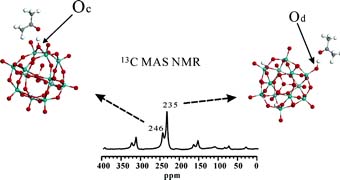
Through solid-state 13C NMR experiments and quantum chemical Density Functional Theory (DFT) calculations of acetone adsorption, they carried out studies into the location of protons in anhydrous 12-tungstophosphoric acid (HPW), the mobility of the isolated and hydrated acidic protons, and the acid strength heterogeneity of the anhydrous hydroxyl groups.
As reported in a recent issue of
Journal of the American Chemical Society, this study presents the first direct NMR experimental evidence that there are two types of isolated protons with different acid strengths in the anhydrous Keggin HPW. Rotational Echo DOuble Resonance (REDOR) NMR experiments combined with quantum chemical DFT calculations demonstrated that acidic protons in anhydrous HPW are localized on both bridging (Oc) and terminal (Od) atoms of the Keggin unit. The CP/MAS NMR experiments revealed that the isolated acidic protons are immobile, but hydrated acidic protons are highly mobile at room temperature. The isotropic chemical shift of the adsorbed acetone suggested that the acid strength of the H(H2O)n+ species in partially hydrated HPW is comparable to that of a zeolite, while the acidity of an isolated proton is much stronger than that of a zeolite. Isolated protons on the bridging oxygen atoms of anhydrous HPW are nearly superacidic.





